|
Ozone and
Photochemical Oxidants
Ozone is not
a pollutant directly emitted into the air from particular activities
characteristic of urban or industrial areas, and can, therefore,
be referred to as a secondary pollutant. Ozone and other photochemical
oxidants (such as peroxyacyl nitrates and aldehydes) are formed
by the action of ultra-violet (UV) light from the sun on
nitrogen oxides (a process called photolysis). Its production
and concentration is dependent on the presence of primary pollutants
as well as ultra-violet light. In the presence of volatile organic
compounds, high concentrations of ozone are formed.
This type of
pollution first gained attention during the 1940's as Los Angeles
smog. Now this smog is described in terms of its photochemical oxidant
composition, which includes ozone and is termed 'photochemical smog'.
Ozone
and Solar Radiation
Ozone is formed
primarily through the action of UV light, and so it could be expected
that high concentrations would occur during the days with high solar
radiation and when other meteorological conditions (e.g. low wind
speed, restricted upward movement of air pollutants) are not favourable
for the dispersion of air pollutants. This relationship between
ozone formation and solar radiation is illustrated by the ozone
levels which occurred in Kwai Chung on 10 July 1994. This can be
seen in the graph below.
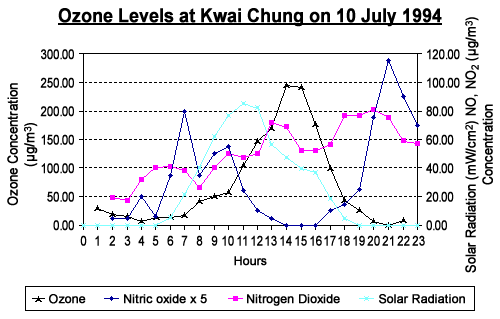 |
The diurnal
variation of the pollutant levels on this very special day started
off with the background levels of nitrogen oxides
and ozone. Automobile and industrial activities began at around
6 am and had the initial effect of increasing the
nitric oxide. The concentration decreased fairly rapidly in
the morning as the high solar radiation, which intensified and peaked
around 11 am, enabled the photochemical oxidation of the
nitric oxide to nitrogen dioxide to occur.
The concentration of nitrogen dioxide reached
a peak at about 1 pm and decreased slightly in favour of ozone,
which reached a peak concentration at about 2-3 pm. As the solar
radiation intensity diminished in the evening, the photochemical
formation of elevated air pollution level ceased and the ozone level
dropped to its background concentration by about 8 pm.
|